Latex Straightjacket Body
€319.90*
Delivery time: 8-10 weeks
-

BeGloss SET 4 - Shine 100 ml -Glide 100 ml - Wash 100 ml
€31.90* -

BeGloss SET 1 - Shine 250 ml - Glide 250 ml - Wash 250 ml
€55.90* -

BeGloss Set 2 - Shine 250 ml Spray - Wash 250 ml - Wipe
€50.90* -

BeGloss Set 3 - Shine 250 ml Spray - Glide 250 ml Spray - Wash 250 ml
€70.90* -

Vivishine Latex Polishing Set 250 ml + polishing pad
€34.90* -

Vivishine Latex Care Set : dressing aid + shine + cleaner + wipe
€55.90*
-

Clean and care for Latex 100ml - beGLOSS
€8.90* -

Clean and care for Latex 250ml - beGLOSS
€12.90* -

Washing lotion for latex and rubber gear
€16.90*
-

Dressing Aid EROS 100 ml
€9.90* -

Latex dressing aid 100ml
€13.90* -

Latex dressing aid 100ml pump spray
€13.90* -

Latex dressing aid 250ml
€22.90* -

Latex dressing aid spray 250ml
€29.90* -

Latex dressing aid from Vivishine -250ml
€21.90*
-

Fetish Latex Shining Spray EROS 150 ml
€14.90* -

Latex high gloss shine spray 100ml
€13.90* -

High gloss latex shine 250ml
€22.90* -

High gloss latex shine 250ml -Spray
€29.90* -

Vivishine -Latex high gloss spray 250ml
€28.90*
Bondage body with mittens and straps
This bondage body has attached mittens with straps. It is also possible to attach a latex mask to this model. The crotch area can either be zipped up or closed as shown.
Shown bondage body:
- Color: white
- Material thickness: 0.40 mm
- Crotch area: closed
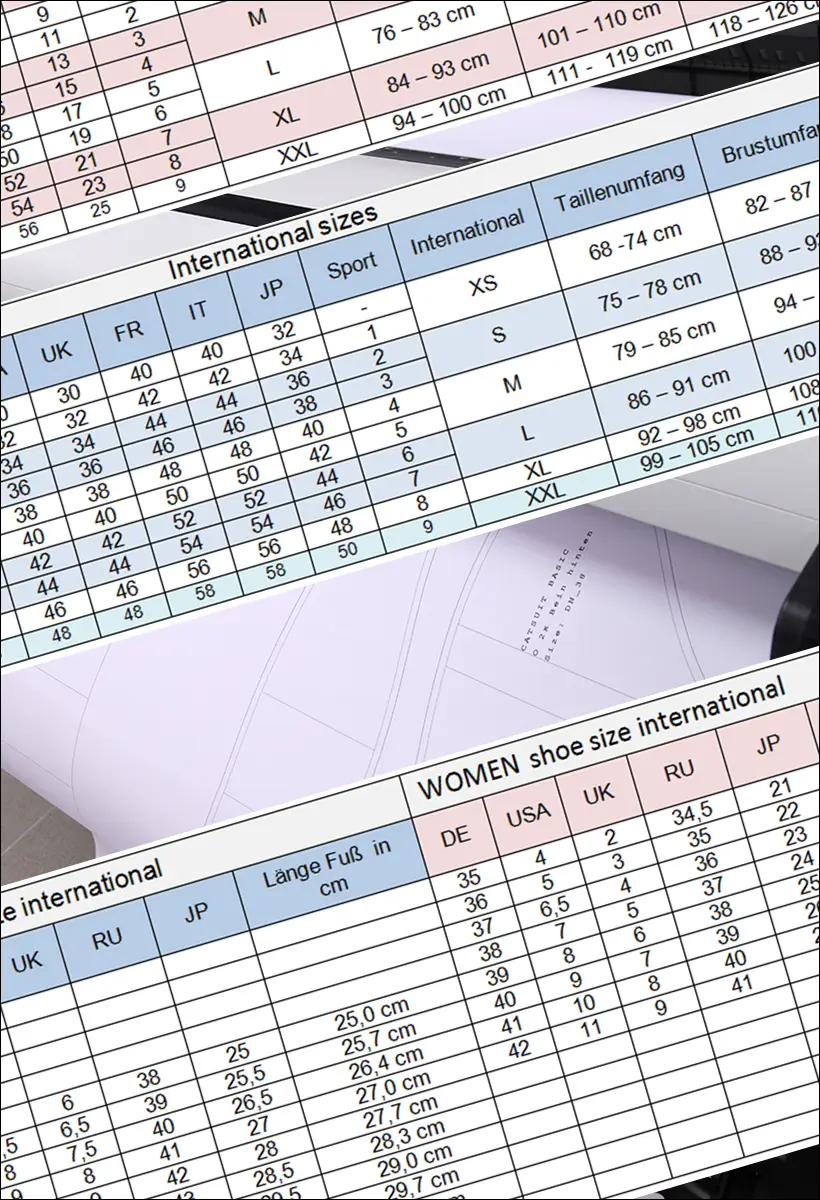
Size charts for latex clothing
The size charts given here are intended as a guide to help you choose the right size for your latex clothing. All sizes are in cm! For information and conversion for our customers with other units of length: 1 inch = 2.54 cm.
The standard sizes of the ready-to-wear sizes for women are based on the average height of a woman from 165 to 175 cm.
The standard sizes for men's clothing sizes are based on the average height of a man from 175 to 185 cm.
Women´s top
| Women | XS-34 | S-36 | M-38 | L-40 | XL-42 | XXL-44 | XXXL-46-48 |
|---|---|---|---|---|---|---|---|
| chest circumference | 78-84 | 84-89 | 89-94 | 94-100 | 100-105 | 105-112 | 112-120 |
| lower breast | 65-70 | 70-75 | 75-80 | 80-86 | 86-92 | 92-98 | 98-106 |
| waist | 57-64 | 64-70 | 70-75 | 75-83 | 83-94 | 94-100 | 100-110 |
| upper arm | 22-24 | 24-26 | 26-28 | 28-30 | 30-32 | 32-34 | 34-36 |
Chlorination of latex clothing
Experience the awesome wearing comfort of our latex clothing by choosing the chlorinated version. Chlorination has nothing done with the shiny surface of the material.
Advantages are simple and clear:
Quick dressing without aid
Chlorination makes the surface smooth and silky. The latex slides on your skin and can be put on without any aid like silicone oil or talcum powder. Therefore chlorinated latex is especially suitable for clothes that are very large and laborous to put on - for example latex catsuits. The effect unfortunately disappears if the skin is clammy or if the skin was previously creamed.
No sticking-together
The latex clothing does neither stick together in the wet state nor in the dry state. This can spare your nerves and faciliate the handling. It is possible to polish the clothes in a tumble dryer using cold air and a soft towel. A basic shininess will appear. Despite this fact, a deep action care with silicone oil is strongly recommended.
Suitable for allergy sufferer:
Via chlorination latex can be conditioned to be suitable for allergy sufferer or to be more suitable than unchlorinated latex. This can be explained with the deterioration of the allergenic protein that is encased in the latex.
Increased life period
Untreated latex features so-called double bonds among the carbon backbone of the material. These bonds can be recognized as "docking site" for radicals which can strongly damage latex or decrease the longevity. During chlorination up to 70% of these double bonds are removed and the chemical durability of your rubber is increased.
But there are also a few disatvantages:
Repairs difficult / impossible
The biggest disadvantage: Only thick latex garments can be sometimes repaired in a satisfactory way by the „grinding method“.
Change of haptics
The material becomes thougher and stiffer. At the beginning the effect can be clearly felt, but over time and by wearing the material it will become softer. Due to the changed haptics the material appears to be of higher thickness than comparable, untreated rubber. A thin catsuit made of 0.25 mm latex will be perceived like a catsuit made of 0.35 mm latex.
The payment is uncomplicated in our store! You can find detailed info about payment and shipping here. We offer the following payment methods:
Once your order has been prepared for shipping, you will receive an email notification with your tracking information. With FedEx Delivery Manager, you now have the option to flexibly redirect your shipment to a pickup station in over 92 countries. This allows you to decide when and where you want to receive your package.
FedEx Delivery Manager is a free online and app service that gives package recipients more control over their deliveries in terms of time, place, and method of delivery. For more information, visit www.fedex.com
INFO: Shipping costs will not be refunded in the event of a return.